ICAR´s contribution to worldwide analytical traceability
On 16 June 2021 a webinar dedicated to the ICAR proficiency test for milk analyses (ICAR PT) was organized. In total, 112 managers and technicians were connected for this specific topic representing DHI and Milk Payment Testing Laboratories of 30 countries.
Dr. Christian Baumgartner chaired the session and introduced the ICAR policy. ICAR as NGO has a key role to promote networking, provide guidance in collaboration with IDF and certify procedures and devices. To practically realize these activities ICAR offers a friendly and collaborative environment, where each member can share and compare experience with the aim to promote analytical equivalence for milk analyses.
In session (A) Dr. Silvia Orlandini presented the new ICAR PT platform. This website allows to subscribe the ICAR PT for the parameters of interest, to upload own results and to download the report with the individual performance and the entire statistical treatment, accredited according ISO 17043.
Session (B) gave an overview of the ICAR PT parameters` precision data. For chemical methods we see that the precision is frequently below the ISO/IDF limits, while the reproducibility of infrared methods is frequently above the ISO/IDF limits.
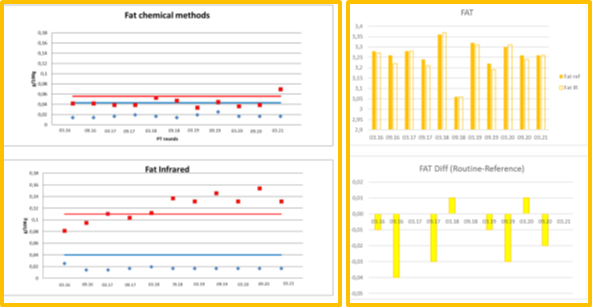 Figure 1. ICAR PT FAT´s precisions and means, comparability of chemical and IR methods
Figure 1. ICAR PT FAT´s precisions and means, comparability of chemical and IR methods
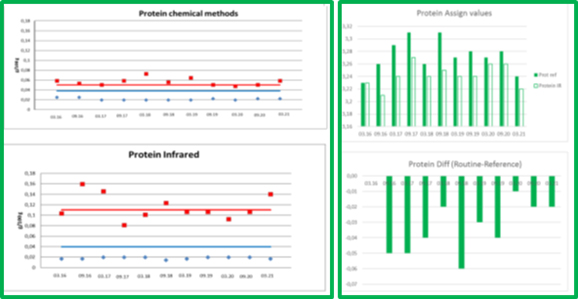 Figure 2. ICAR PT PROTEIN´s precisions and means, comparability of chemical and IR methods
Figure 2. ICAR PT PROTEIN´s precisions and means, comparability of chemical and IR methods
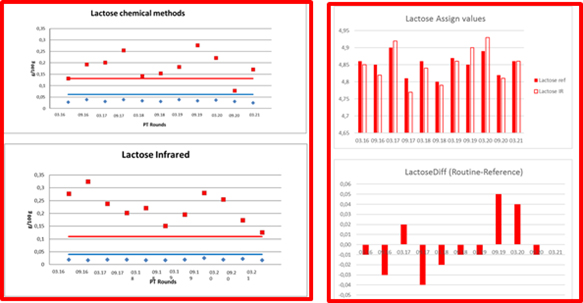 Figure 3. ICAR PT LACTOSE´s precisions and means, comparability of chemical and IR methods
Figure 3. ICAR PT LACTOSE´s precisions and means, comparability of chemical and IR methods
 Figure 4. ICAR PT UREA´s precisions and means, comparability of chemical and IR methods
Figure 4. ICAR PT UREA´s precisions and means, comparability of chemical and IR methods
The comparison of the chemical assigned value and routine assigned value identified for total nitrogen content (protein) shows a constant infrared underestimation (see Figure 2).
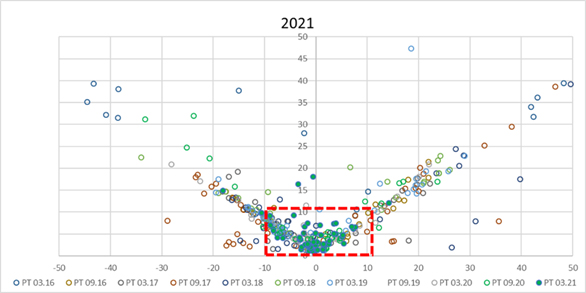
The third session (C) was specifically dedicated to the somatic cell counting performance and traceability to the certified reference material EC JRC CRM SCC. Figure 5 shows clearly how in the last PT round in March 2021, after one year of the EC JRC CRM SCC availability, the equivalence between the ICAR PT participants improved dramatically. The existence and use of the EC JRC CRM SCC is one of the most relevant fruits of the IDF/ICAR project Reference System for SCC (RSSCC)
Figure 5. SCC – Laboratory performance in past ICAR PTs. Blue bold dots represent the ICAR PT March 2021
Questions/Comments from the audience
It was requested to check the interest among ICAR and non-ICAR labs to organize an ICAR PT for goat and camelid milk. Furthermore, questions regarding the unit of measurement for urea were put forward. Finally it was asked to try to improve the delivery time of PT materials to Canada and Argentina.
Conclusions
From the objective data available and presented during this webinar, we can conclude that ICAR and IDF collaboration is a strategic tool to obtain global equivalence for milk analyses. This collaboration will be continued in the future, investing even more efforts in applying the structure of Reference Systems to other parameters.