The devices used by ICAR Member Organisations for official milk recording purposes must be ICAR-certified, as stipulated in Section 11 of the ICAR Guidelines. This section outlines the rules, standards and recommendations for testing, certification and periodic checking of milk recording and sampling devices.
The full text of Section 11 may be found here.
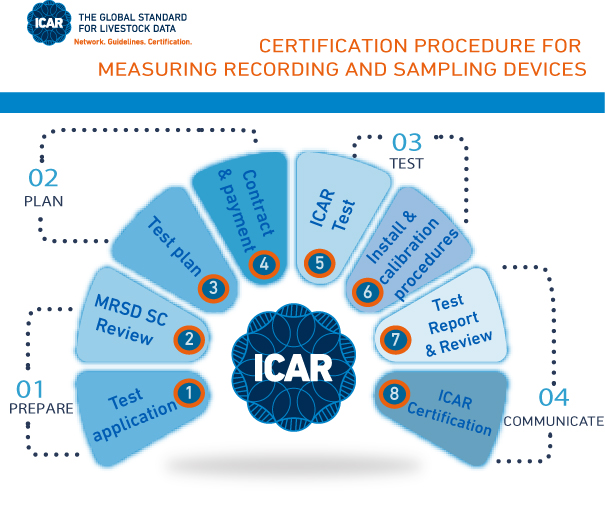
The process for obtaining ICAR certification is as follows:
- The applicant submits a test application form.
- The application is reviewed, and the Test Centre is designated
- The Test Centre prepares the test plan, detailing the timeline and associated costs.
- ICAR provides the applicant an umbrella contract and invoice for test fees, along with the test plan.
- Testing begins upon signing of the contract by the applicant and full payment of the test fees.
- Upon test completion, ICAR disseminates the report to the MRSD Sub-Committee for review, comments, and recommendations.
- ICAR forwards the report to the applicant and issues the official ICAR Certificate upon successful completion of the test.
- The certified device is listed on the ICAR website.
- All ICAR-certified devices intended for market distribution must have labels issued by ICAR. Label requests should be directed to yvonne@icar.org.
For technical information, please contact Mr. Steven Sievert, Chair of the ICAR MRSD Sub-Committee, at sjsievert@dhia.org or at +1 608 444 1285.
The ICAR Secretariat contacts are:
Process issues – application, test procedure, certification:
Mrs. Andie Dimitriadou
Information & Knowledge Senior Executive
andie@icar.org
Tel.: +39 334 8420774
Contracts, payments, label orders:
Mrs. Yvonne de Gier
Finance & Operations Senior Executive
yvonne@icar.org
Tel. +31 6 4842 9118
